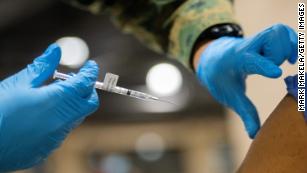
The FDA has expanded the emergency use authorization for Pfizer’s Covid-19 vaccine to include people ages 12 to 15. This is the first Covid-19 vaccine in the United States authorized for use in younger teens and adolescents; the vaccine had previously been authorized for people age 16 and older. Covid-19 vaccines from Moderna and Johnson & Johnson are authorized for use in people age 18 and older.
Read the full article:
CNN